Summary of research progress in the field of liver diseases (02.09)
Summary of research progress in the field of liver diseases (02.09)
February 11, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];1. Liver disease ultrasound medical imaging system obtained FDA approval
SuperSonic Imagine, a company specializing in ultrasound medical imaging, recently announced that the 510(k) report of the Aixplorer and Aixplorer Ultimate ultrasound diagnostic systems has been approved by the US FDA to assist in the clinical management of patients with liver disease. 510(k) is a pre-marketing report submitted to the FDA to demonstrate that the safety and effectiveness of the equipment to be sold is comparable to other equipment that has been approved for sale. This license provides doctors with new and effective clinical indicators that can be used for non-invasive assessment of liver fibrosis and steatosis using Aixplorer.

According to reports, in 2015 alone, 3.9 million American adults were diagnosed with liver disease, and more than 38,000 people died. Chronic liver disease increases the risk of primary liver cancer. In 2014, more than 31,000 Americans were diagnosed with liver cancer, and about 25,000 people died of liver cancer in the same year. In addition, according to 2014 data, 38% of American adults are obese and 12% are diagnosed with diabetes. Both of these conditions increase the risk of nonalcoholic steatohepatitis (NASH), a hallmark of fat accumulating in the liver and inflammation and fibrosis. The prevalence of NASH in some parts of China can be as high as 27%. The number of NASH patients in the United States is also as high as 16 million. In the absence of effective treatment, NASH may induce severe liver problems including liver cancer.
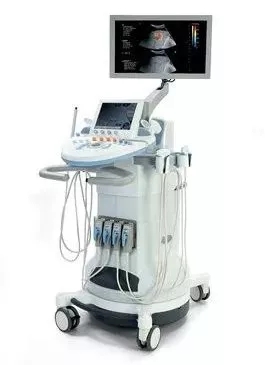
â–² The Aixplorer system provides routine morphological and hemodynamic imaging, as well as real-time rapid imaging using the platform's SWE technology to measure the stiffness of the liver and spleen. (Source: SuperSonic Imagine)
The Aixplorer system provides routine morphological and hemodynamic imaging. In addition, the system uses ShearWaveTM Elastography (SWE) technology for real-time rapid imaging to measure liver and spleen stiffness. It is known that the stiffness of the liver and spleen is related to the severity of liver fibrosis, so this indicator is considered to be a key non-invasive marker of disease severity. The doctor can also use the Aixplorer system to compare the ultrasound liver brightness to the reference tissue, thereby obtaining a liver-kidney brightness ratio that indicates the degree of hepatic steatosis. In addition, Aixplorer can visualize and quantify abdominal vascular perfusion, an ability that will aid in the clinical management of patients with liver nodules and advanced chronic liver disease.
"Aixplorer and Aixplorer Ultimate's ability to measure tissue stiffness is revolutionary, and their color-coded images provide a safe, accurate, non-invasive biopsy alternative in 60 seconds," Baylor University, Dallas, Texas, USA Dr. Sumeet Asrani, a liver specialist at the Baylor University Medical Center, commented, “From a diagnostic point of view, these advances are exciting, they speed up the diagnosis, reduce the need for invasive biopsy, and thus improve chronic liver disease. The clinical experience of the patient."
2. Clinical trial of new drug for children with liver disease
Gemphire Therapeutics is a biopharmaceutical company developing cardiovascular and metabolic disease therapies focused on the development and commercialization of therapies including diseases such as dyslipidemia and nonalcoholic steatohepatitis (NASH). The company recently announced the launch of a proof-of-concept phase 2a clinical trial to evaluate the efficacy of gemcabene as a nonalcoholic fatty liver disease (NAFLD) therapy in children. The trial is part of a larger plan to evaluate gemcabene in patients with NAFLD/NASH and will be conducted in parallel with recent studies of familial partial lipodystrophy (FPL) testing for gemcabene. The top line results of the pediatric NAFLD trial are expected to be announced in early 2019.

NAFLD is becoming the most common chronic liver disease in many countries. There are approximately 75 to 100 million NAFLD patients in the United States, which is the leading cause of chronic liver disease in children and adolescents in the United States. It is also a major burden of chronic liver disease in China. It covers everything from the mildest liver fat infiltration to simple steatosis (NAFL) to more severe NASH and liver fibrosis. Its treatment is very limited.
Gemphire's drug candidate gemcabene is expected to change this situation. It is a first-in-class, once-a-day oral therapy that may be suitable for use with currently approved therapies (primarily statins) but low-density lipoprotein cholesterol (LDL-C) or triglycerides still do not reach normal levels Patient. Gemcabene aims to increase the clearance of very low density lipoprotein (VLDL) in plasma and inhibit the production of cholesterol and triglycerides in the liver.
The publicly-labeled clinical trial will recruit approximately 40 children between the ages of 12 and 17 who have been diagnosed with NAFLD and abnormal liver function by liver transaminase assessment. The patient will receive a 300 mg dose of gemcabene once a day. The primary endpoint was the change in serum alanine aminotransferase (ALT) from baseline to 12 weeks, and ALT is an enzyme for liver function biomarkers. Secondary endpoints included changes in hepatic steatosis measured by non-invasive magnetic resonance imaging-proton density fat fraction (MRI-PDFF), changes in liver inflammation and liver fibrosis (LIF) scores by non-invasive MRI, changes in AST , insulin sensitivity, changes in blood lipids (including triglycerides), apolipoproteins and inflammatory markers (including hsCRP), as well as drug safety and tolerability.

â–² Dr. Steven Gullans, Interim President and CEO of Gemphire (Source: Gemphire)
Dr. Steven Gullans, Interim President and CEO of Gemphire, said: "We are very excited to launch this pediatric clinical trial as a next step in evaluating the efficacy of gemcabene in adult and adolescent NAFLD/NASH patients. We believe that based on its good safety and Gemcabene will have a clear competitive advantage in response to new mechanisms of dyslipidemia and inflammation."
3. Study on the treatment of rare liver disease with cannabidiol
Revive Therapeutics has announced a study on the treatment of autoimmune hepatitis (AIH) with cannabidiol (CBD). The study will be overseen by the company's strategic partner for liver disease, Sanyal Biotechnology.

AIH is a rare autoimmune disease that can cause inflammation of the liver. If not treated properly, it can lead to liver fibrosis, cirrhosis, liver failure and even death. It is estimated that there are 75,000 AIH patients in the United States. Current AIH standard care is the use of steroids alone, or the combination of steroids with azathioprine. However, according to statistics, using this standard for a period of time may result in 13% of patients with severe treatment-related side effects, 9% of treatment failures, 13% of incomplete remission, and up to 86% recurrence rate after discontinuation. Therefore, treatment options that do not contain steroids (such as CBD) may provide improved treatment strategies for AIH patients.
The research project will be led by Sanyal's scientific team, based on the DIAMONDTM model developed by Sanyal, to create a new AIH model for CBD treatment. The research project is expected to produce a better AIH model, allowing Sanyal to further promote the use of CBD to treat AIH and other liver diseases, and will provide meaningful data for Revive's future clinical research.

Mr. Fabio Chianelli, President of Revive, said: "We are very pleased to be able to use Sanyal's biotechnology to promote the development of AIH using CBD. This research will not only serve as a basis for future clinical research on AIH, but also to expand our treatment of rare liver diseases. Knowledge and lay the Foundation for market opportunities for large liver diseases such as nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH) and liver fibrosis."
Reference materials:
[1] FDA Green Lights SuperSonic Imagine's Aixplorer and Aixplorer Ultimate Liver Disease Ultrasound Diagnostic Systems
[2] Gemphire Announces Initiation of Phase 2a Clinical Trial of Gemcabene in Pediatric Non-Alcoholic Fatty Liver Disease (NAFLD)
[3] Revive Therapeutics Advances Research Program of Cannabinoid-Based Therapies Targeting Liver Diseases
Original Title: Summary of Research Progress in the Field of Liver Diseases (No. 33)
Foundation
Foundation,The Foundation,Forward The Foundation,Foundation'S Edge
Guangzhou Lingxue Cosmetics Co., Ltd , https://www.gzlxgj188.com