Leica Classroom | Leica, the invisible scissors that help you master genetic editing - the application of micromanipulation technology in gene editing
This article will review the micromanipulation techniques in the field of mouse transgenes, including CRISPR/Cas9 technology, to introduce the workflows and terminology used by such customers. In addition, some suggestions are provided for useful microscopy operating system configurations.
Micromanipulation Technology Overview
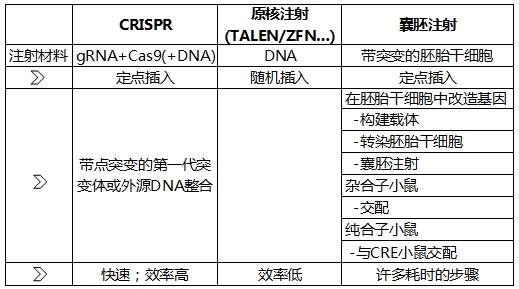
Figure 1: A rough comparison of CRISPR/Cas9, pronuclear injection and embryonic stem cell transplantation techniques. See below for more details.
Early stage of mouse embryo development
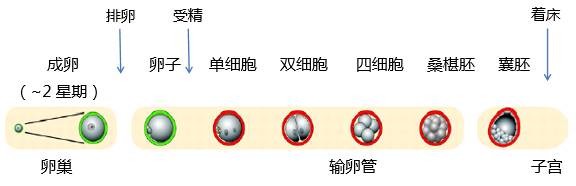
Figure 2: Schematic diagram of the early developmental stages of mouse embryos (night fertilization, morning injection.) (Source : http://dev.biologists.org/content/137/6/859)
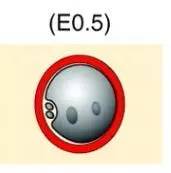
Figure 3: Schematic diagram of the pronuclear stage (transparent band marked in red)
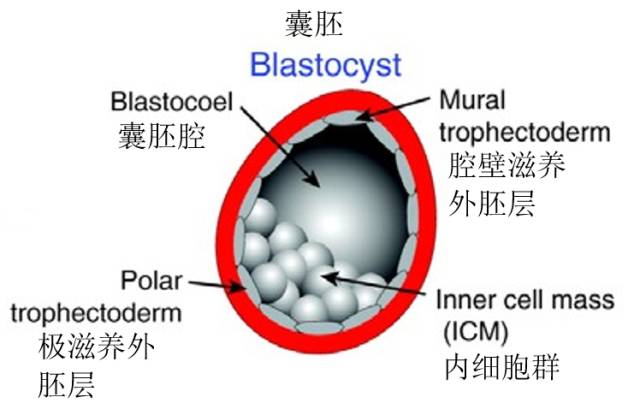
Figure 4: Schematic diagram of blastocyst structure
Micromanipulation technique
Pronuclear injection (PNI) and embryonic stem cell transplantation (ESI)
Pronuclear injection/fertilization egg injection techniques or embryonic stem cell injection techniques are used regardless of whether or not CRISPR (or TALEN, ZFN (see Chapter 4)) is involved. These two technologies are mature mainstream technologies that are used worldwide:
Pronuclear injection
About 12 hours after fertilization, an egg pronucleus and a sperm pronucleus appeared (Fig. 2). Before the prokaryotic fusion is integrated and constitutes the nucleus of the fertilized egg, the DNA is injected into a pronucleus in the fertilized egg of the pronuclear stage, and has a diameter of about 80-100 μm. The injected target DNA is randomly integrated into genomic DNA. The direction and copy number are unpredictable, ie, this is a non-targeting technique, and the probability of obtaining a transgenic mouse of interest quickly and directly is very limited.
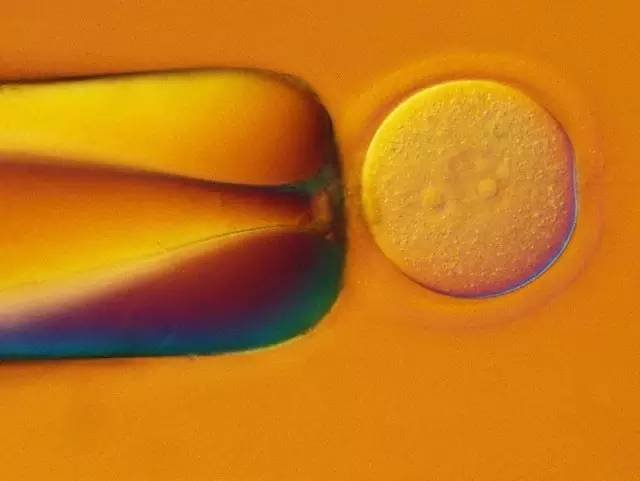
Figure 5: PNI. The mouse embryo of the PN stage (left side) was fixed with a fixed capillary. A small amount of DNA dissolved in the buffer was injected into one of the pronuclei (right side) via a capillary. It can be seen that the injected pronucleus expands. The picture was collected with Leica DIC.
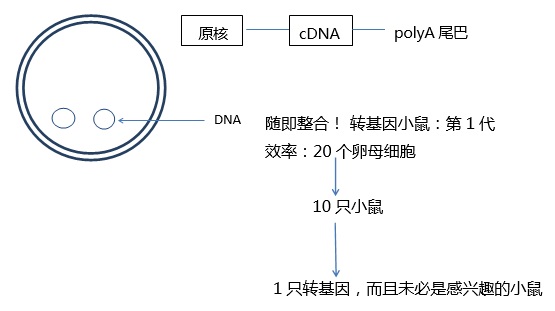
Figure 6: Random non-targeted integration of DNA into the genome during PN injection
Typical PNI system settings:
-DMi8: Manual, electric parts are available
- transmitted optical axis; S28 concentrator and differential interference difference
-5/10 times objective lens (FLUOTAR or N PLAN) for general observation
-20 times, 40 times long working distance objective lens. 40x objective for injection
-3 onboard stage (object guides on the fixed stage obstruct the micromanipulator!)
- Select micromanipulators (preferably direct injection of capillaries: flat angles help to minimize embryo damage)
- Pneumatic manual microinjector for fixation (negative pressure generated by counterclockwise rotation can fix the embryo)
- FemtoJet® microinjector (small liquid, pressure x time = volume)
- Select sample carriers (glass bottomware, coverslips, etc.) according to laboratory preferences
PN injections are typically performed at room temperature by means of a 400-fold differential interference contrast microscope (some also using IMC). (In some cases, the embryo is cooled to 10 ° C to make the plasma membrane stiffer to facilitate injection)
Embryonic stem cell transplantation
Embryonic stem cells are injected into the blastocyst (Fig. 4), and the blastocyst stage starts from day 3.5 with about 128 cells. The inner cell mass is composed of pluripotent embryonic stem cells, that is, they can develop and differentiate into different types of cells. Sometimes eucalyptus embryos are injected into the eight-cell stage (Figure 9).
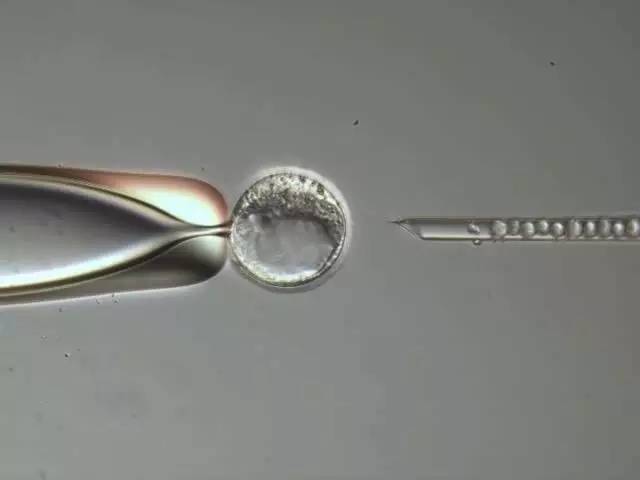
Figure 7: ESI. The mouse embryo sac was fixed with a fixed capillary (left side). The genetically modified embryonic stem cells are injected into the blastocyst cavity (right side) via a capillary. Injection is done between the inner cell population and the trophectoderm cells. The picture was taken with the Leica DIC on the Leica AM6000.
The injected embryonic stem cells and embryonic stem cells of the inner cells develop into mice. This is a chimeric mouse whose cells/organs are derived from injected embryonic stem cells (male) and embryo sac (male/female).
Editing of the gene of interest is done prior to embryonic stem cell transplantation, which takes about 3-4 months until the ES cell clone carrying the genomic DNA target sequence is selected. Most of the time the modification appears only in one allele, meaning that the chimera is heterozygous. The resulting clone carries a target mutation on one allele (the probability on both alleles is very low). This technique allows the insertion of approximately 10-20 kB of DNA. The method used for targeting mutations is homologous recombination.
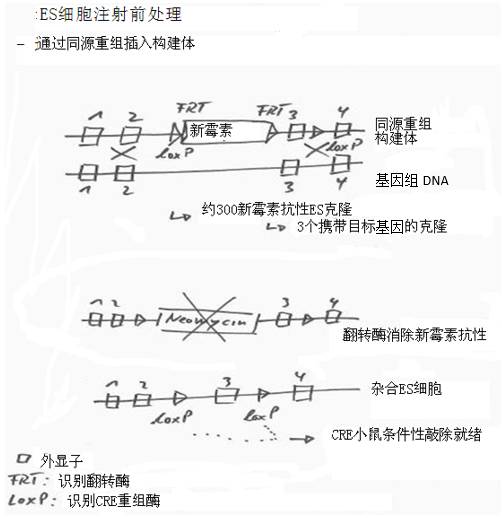
Figure 8: Targeted integration/mutation of ES cell DNA
1) Using DNA biology techniques to create DNA containing target DNA as well as neomycin resistance sites, flip-flop recognition sites (FTR) and CRE recombinase recognition sites (loxP).
2) Injecting target DNA into ES cells by transfection. The recombinant is integrated into genomic DNA by homologous recombination.
3) Add neomycin (an antibiotic) to the cell culture to select the ES clone produced. Only clones with neomycin resistance can survive. Pick out these clones and continue to grow.
4) Add a flipping enzyme (a recombinase that cleaves DNA and recombines it) to eliminate neomycin resistance.
5) The loxP site can be conditionally knocked out by transgenic mice mated with another transgenic "CRE mouse". Mice expressing CRE recombinase typically have tissue-specific expression that confers targeted knockdown of genes in the brain, for example.
Targeted mutations via homologous recombination and ES cell injection have been the "gold standard" for many years. In contrast to CRISPR, the deficiencies include:
Long carrier preparation time
Select ES cell clone time
Vector cloning and ES cell work takes about 3 months
It takes about 1 year to generate homozygous mice.
As mentioned above, in approximately 99% of cases, the chimeras produced were heterozygous. The ultimate goal is to obtain homozygous animals for research.
This is a time-consuming and laborious mating and screening process:
1) Embryo sac (♀ or ♂) + ES cells (♂) produce ♂ chimera. The aim is to obtain chimeras with ES cell modifications in germ cells.
2) æ‚ Hybrid chimera + WT ♀ produces heterozygous F1 (♂ or ♀)
3) Heterozygous ♂ + Heterozygous 产生 Produce homozygous F2 generation
Both homozygous mice carry mutations on both alleles. It takes about a year to get these valuable animals!
Typical ES porting system settings:
-DMi8: manual and electric parts are available;
- transmitted optical axis; S28 concentrator
-DIC is not required. The embryo is large enough at the embryo sac stage and its details are easy to observe.
- 5/10 times; 20 times; 40 times long working distance objective lens; smaller NA = larger depth of field
-3 onboard stage (object guides on the fixed stage obstruct the micromanipulator!)
- Select micromanipulators (preferably direct injection of capillaries: flat angles help to minimize embryo damage)
-Pneumatic manual microinjector for fixing
- Hydraulic or hydraulic geared manual microinjector for injection of ES cells.
ES cell injection is typically done at room temperature with a 200x magnification DIC/IGC/PH microscope.
The difference in ESI is that ES cells are injected into embryos of the 8-cell stage (Figure 9). This is usually done using a blunt-ended capillary to avoid damage to the cells. A laser (Hamilton-Thorne, Octo or Piezo Piezoelectric Membrane Breaking System) helps the blunt end capillaries pass through the zona pellucida and the membrane.
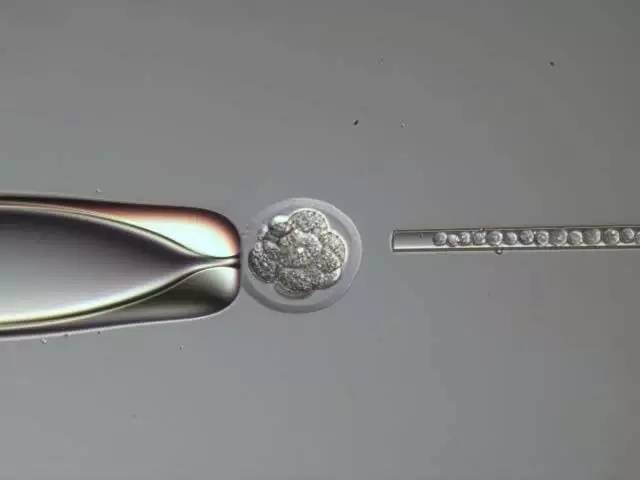
Figure 9: Embryos injected into the 8-cell stage of ESI. Blunt end capillary
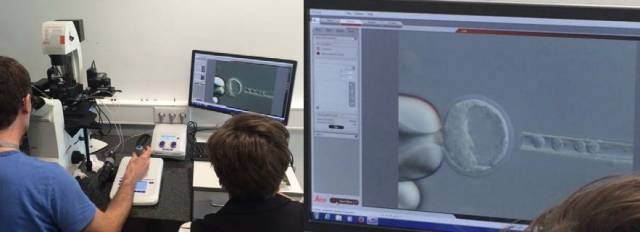
Figure 10: DMi8, Eppendorf TransferMan 4r micromanipulator and PiezoXpert piezoelectric membrane disruptor used in the EMBL transgenic process. The blastocyst injection was performed with a blunt capillary. DIC
CRISPR/Cas 9 technology for mouse transgenes
The CRISPR/Cas9 technology for mouse transgenes provides tools (such as ESI) for targeted mutations, but does not require complicated ES processing! (Of course, CRISPR can also be used for ES cell mutations).
Transcriptional activator-like effector nuclease (TALEN) technology is a more mature technology. TALE (transcriptional activator-like effector) is a protein that binds to a specific sequence of DNA. The fusion of TALE and nuclease is TALEN, a highly specific DNA scissors. TALEN breaks the double strand. The repair mechanism results in deletion or knockout of the target gene.
Currently, CRISPR technology is not expensive relative to TALEN technology.
Another genomic editing technique is the zinc finger nuclease technology, which works by causing DNA double-strand breaks at a user-specified location.
The tools used in CRIPR/Cas9 technology are as follows:
Guide RNA : This RNA is homologous to the user-modified target DNA. The guide RNA binds to approximately 20 nucleotides on the DNA. The remainder of the RNA strand is used to bind to the Cas9 endonuclease. Twenty bound nucleotides need to be designed to find/find the target sequence.
Cas9 endonuclease : high cleavage efficiency, sometimes even cutting two alleles.
The guide RNA and Cas9 protein (or Cas9 DNA or mRNA) are injected into the fertilized egg, Cas 9 cleaves the DNA, and the repair mechanism begins to function. This method has a high probability of producing point mutations in the first generation of mice. A more advanced technique is to inject homologous DNA carrying the target gene or mutation and a guide RNA/Cas9 mixture. Insertion of DNA via homologous recombination is quite effective.
Advantages/disadvantages of mouse transgenic with CRISPR/Cas9:
advantage:
+ Cas 9 cuts very efficiently, sometimes even cutting two alleles to get a homologous 1 generation mouse!
+ No need for cumbersome ES cell processing
+ CRISPR/Cas9 technology is not dependent on mice (and PNI and ESI are dependent), ie it can also be used in other species (pig, etc.).
Disadvantages:
- The longest insert that has been published so far is 3kB
- Currently low efficiency of lox P conditional knockout
The need to inject high concentrations of viscous molecules (such as RNA, protein), will block the capillary. Therefore, a thicker capillary is required, and a flat angle injection is required!
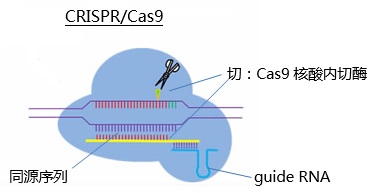
Cas9 cuts DNA efficiently, and the DNA repair mechanism causes point mutations.
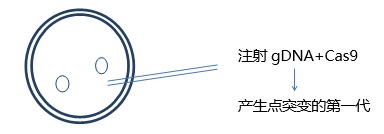
*Inject gDNA+Cas9+ homologous DNA
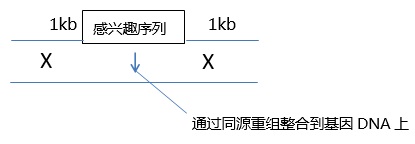
Figure 11: CRISPR/Cas9 Technology
Typical CRISPR/Cas9 system settings
Same as PN injection settings, except the following:
Thicker capillaries are required to inject higher concentrations of viscous CRISPR components (RNA, protein) into fertilized eggs. These capillary structures are straight and have a large internal diameter and are manufactured in most laboratories.
It is therefore highly recommended to inject along the true flat angle (see Figure 10) to avoid embryo damage.
In addition, the Piezo Piezo Film Breaking System makes it easier to pass through the transparent belt, especially the plasma membrane.
The micromanipulator moves horizontally and injects the target fragment into the suspended cells.
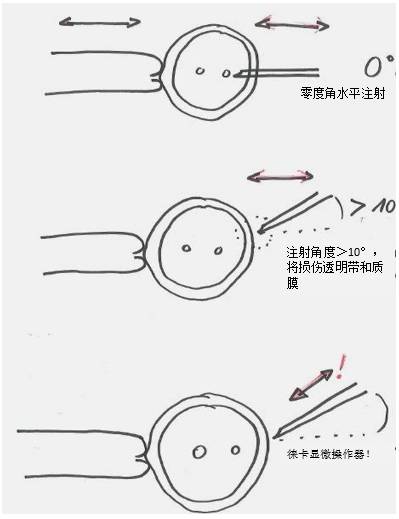
Figure 12: Injection angle of the fertilized egg injection process. The micromanipulator moves horizontally, and a zero-degree angle injection can make the embryo less damaged. An injection angle greater than 10° will result in a larger “hole†in the fertilized egg, which will reduce its survival rate.
The movement angle of the Leica micromanipulator is adjustable, that is to say, even if the angle is steep, the injection angle itself is zero angle!
This is one of the great advantages of the Leica Micromanipulator: the thicker capillary (reducing clogging) required for the viscous guide RNA and the cas9 protein mixture can be injected along the zero angle!
Other laboratory equipment (genetically modified laboratory)
- Stereomicroscopy is an essential tool for selecting and controlling embryo sacs, ES cells and capillaries. (eg TL5000, M80, Rottermann contrast)
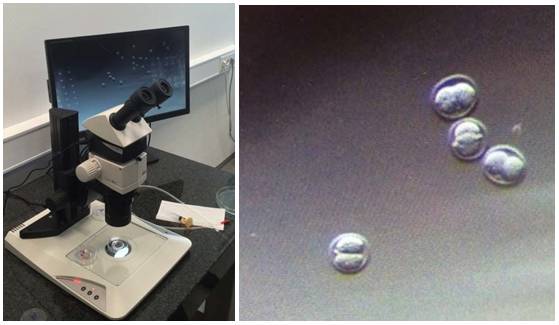
Figure 13: M80 and TL5000 Ergo. The two-cell stage after PN injection.
DIC special glass bottomware, some labs use large coverslips
Capillary: Several companies offer out-of-the-box products. Many transgenic laboratories make their own capillaries.
Straight-line injection of CRISPR/Cas9 requires the use of capillaries with a sufficiently large internal diameter. Genetically modified experts make their own "advanced" capillaries for maximum efficiency.
Need tools:
Needle puller (eg Sutter, Narishige)
Abrasives (for capillary drawing and processing)
Micro-drawing instrument (for bending capillary)
The above micromanipulator :
Leica Mechanical Micromanipulator : A highly acclaimed type of micromanipulator in the field of genetic modification. The angle is adjustable, and even if the capillary angle is steep, a zero injection angle can be obtained. Robust and reliable, with strong load capacity and durability. There are many users. When the lever of the Leica micromanipulator is moved, the capillary can be felt to touch the embryo.
Eppendorf TransferMan4r : Fully automatic micromanipulator with many additional features. Can be injected at a flat angle. Pneumatic, hydraulic, hydraulic geared and Femtojet manual microinjectors are often used in other micromanipulators. The best performance and the highest price.
Narishige : The new Takanome micromanipulator has no flat angle operation. With MOM-202D and MON-202D, it can be operated at a flat angle. Oil-operated micromanipulators are of course second to none. Syringes: IM 9B, IM 9C, IM-11, IM300.
Shockproof: It depends to a large extent on whether the injection chamber is located in the basement or on the tenth floor of a high-rise building (whether or not it is needed). Passive (iron plate, heavy stone table, tennis, etc.) and active settings.
Leica DMi8 transgenic system features
• Highly modular inverted research microscope – manual/electrical components are available upon request
• Low height of the stage – better ergonomics and safer embryo handling
• Microscope stable – less vibration
• Perfect Leica optical system!
• Best DIC! – original nuclear visualization
• Intelligent Automation – use/deactivate optics with the click of a button
• Integrated modulation and phase contrast – standard objective without modulator / PH ring
• Miniature Leica Micromanipulator Console (located close to the microscope, so close to the optical axis/sample)
• The Leica operator is always injected at zero angle!
• Leica Micromanipulator: Reliable, precise, and well-known
Compatible with Eppendorf and Narishige micromanipulators
references
One-Step Generation of Mice Carrying Mutations in Multiple Genes by CRISPR/Cas-Mediated Genome Engineering
Haoyi Wang, Hui Yang, Chikdu S. Shivalila, Meelad M. Dawlaty, Albert W. Cheng, Feng Zhang, Rudolf Jaenisch, Cell 153, 910-918, May 9, 2013
-Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system
Albert W Cheng, Haoyi Wang, Hui Yang, Linyu Shi, Yarden Katz, Thorold W Theunissen, Sudharshan Rangarajan, Chikdu S Shivalila, Daniel B Dadon and Rudolf Jaenisch, Cell Research (2013) 23:1163–1171. doi:10.1038/cr .2013.122; published online 27 Aug 2013
Eas Safer Box,Security Box For Cosmetic ,Clear Plastic Lock Box,Magnetic Lock Box
ZHEJIANG BOSHINE ELECTRONIC SECURITY CO.,LTD , https://www.boshine.com