Analysis of the registration of medical device products in China in the past ten years
On May 23, 2017, the State Food and Drug Administration issued the 2016 annual report on food and drug supervision statistics. The circle of friends of Xiaobian has been smashed by the information point of “increasing more than 100,000 medical device operators nationwideâ€. But in addition to the number of companies, the number of medical device registrations in recent years has not attracted attention. Xiao Bian made a statistical analysis of the medical device registration data from the first period to the latest issue of the regulatory statistics annual report, from the registration volume to see the changes in the medical machinery circle in recent years:

According to the 2016 annual statistical report, the country approved a total of 11,539 domestic first-class medical devices, 3,023 imported first-class medical devices, 6,093 domestic second-class registered medical devices, and 929 domestic third-class registered medical devices. Imported 444 registered medical devices (including Hong Kong, Macao and Taiwan) and imported 593 registered third-class medical devices (including Hong Kong, Macao and Taiwan). Approved the second category of continuation of registered medical devices in China, 6076, the third category of continuation of registered medical devices in China, and the import of second-class continuation of registered medical devices (including Hong Kong, Macao and Taiwan) 2,077, and the import of third-class continuation of registered medical devices (including Hong Kong and Macao) Taiwan) 1634 pieces. In the second category of licensing matters, 3,814 medical devices were changed, and 1,466 medical devices were changed in the third category of domestic and imported (including Hong Kong, Macao and Taiwan) licensing matters. (See Table 1)
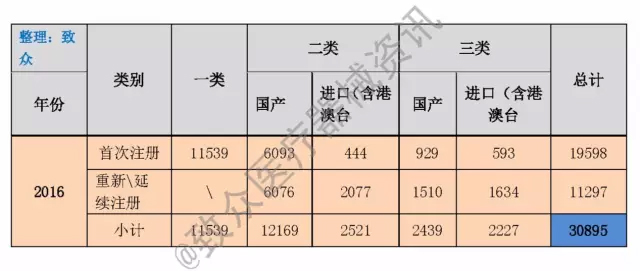
Table 1 2016 Medical Device Registration Form
As of this year, the State Food and Drug Administration has issued 11 statistical annual reports, the data comparison is as follows (see Table 2):
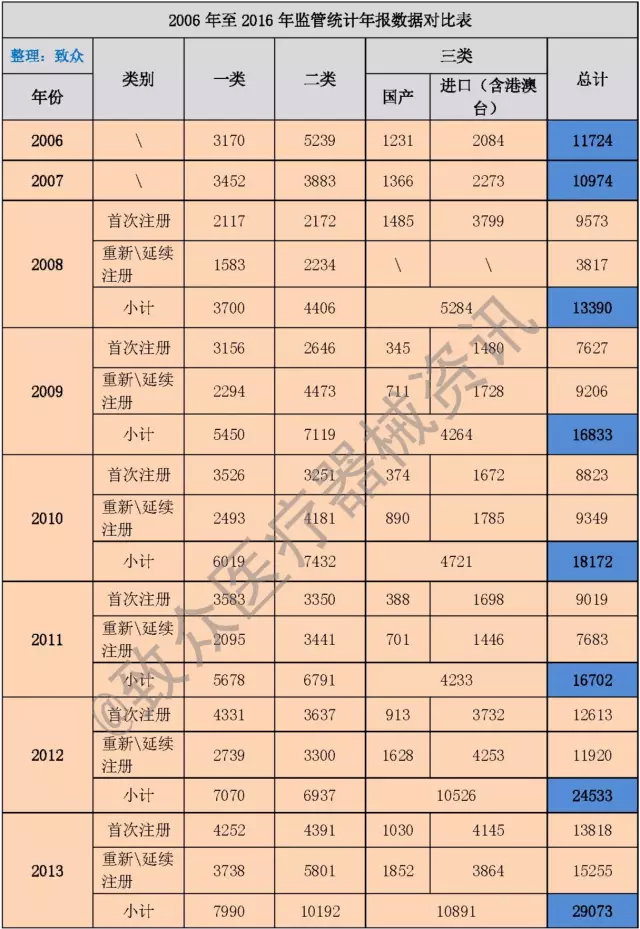
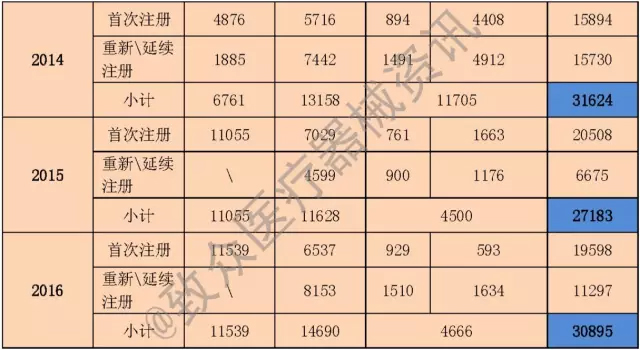
Table 2 Comparison table of regulatory statistics annual report from 2006 to 2016
From the total number of registrations, the total volume of the medical device market has generally increased steadily. Only in 2015, due to factors such as the implementation of new regulations, there has been a significant decline (see Figure 1).
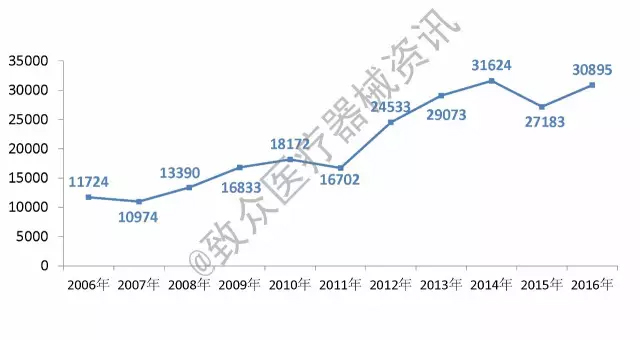
Figure 1 Trends in the total number of medical device registrations
Nutritional supplements, also known as nutritional supplements, nutritional agents, dietary supplements, etc., are used as an auxiliary means of diet to supplement amino acids, trace elements, vitamins and minerals needed by the human body. Nutritional supplements can be composed of amino acids, fatty acids, minerals and vitamins, or only consists of one or a variety of vitamins, can also be composed of one or more dietary ingredients, in addition to including amino acids, vitamins, minerals and other nutrients, also can have herbs or other plant components, or more ingredients of concentrate, extract or combination.Nutritional supplements, also known as dietary supplements, nutritional supplements, nutritional agents, dietary supplements, etc., are used as an auxiliary means of diet to supplement amino acids, trace elements, vitamins and minerals needed by the human body.we have Alpha GPC 50%,Tototrienol,7 8-Dihydroxyflavone,Palmitoylethanolamide Granular ,RU58841,1-1-Adamantylcarbonyl proline ACA,Galantamine Hydrobromide,Nooglutyl,Tianeptine Sodium,Oleoylethanolamide OEA,Sunifiram,Oleamide ODA,N-Dodecanoyl-L-Proline.
Alpha GPC 50%,Tototrienol,7 8-Dihydroxyflavone,Palmitoylethanolamide Granular,RU58841.
Xi'an Henrikang Biotech Co.,Ltd , https://www.henrikangbio.com