Three types of medical devices, such as electronic thermometers, failed sampling
Recently, the State Food and Drug Administration has organized quality supervision and sampling of products of 247 batches (sets) of three varieties, including disposable tracheal intubation and medical electronic thermometers.
The medical device products that are not in compliance with the standard are required to be sampled, involving 3 varieties and 4 batches (sets) of 4 medical device manufacturers. Specifically:
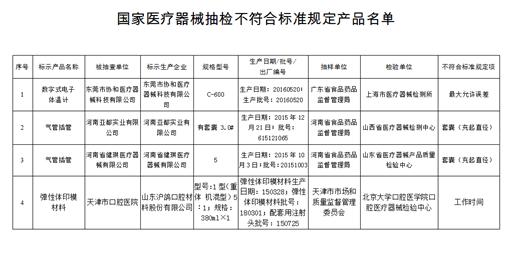
Medical electronic thermometer 1 company, 1 product. A digital electronic thermometer produced by Dongguan Xiehe Medical Device Technology Co., Ltd., the maximum allowable error does not meet the standard;
One-time use of tracheal intubation in 2 companies of 2 companies. Each batch of tracheal intubation produced by Henan Yadu Industrial Co., Ltd. and Henan Jianqi Medical Devices Co., Ltd., the cuff (filling the diameter) does not meet the standard;
Elastomer impression material 1 company 1 batch of products. One batch of elastomeric impression materials produced by Shandong Huge Dental Materials Co., Ltd. does not meet the standard requirements for working hours.
The sample items to be inspected are medical device products that do not meet the standards as specified in the label, manual, etc., and involve 2 varieties of 2 medical device manufacturers, specifically:
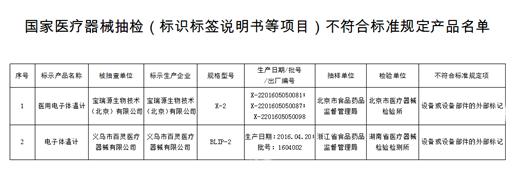
Medical electronic thermometer 2 companies 2 products. The external marking of an electronic thermometer, equipment or equipment parts produced by Baoruiyuan Biotechnology (Beijing) Co., Ltd., a medical electronic thermometer and Yiwu Bailing Medical Instrument Co., Ltd. does not meet the standard.
All the sampling items that meet the standard requirements include 241 batches (sets) of 3 varieties of 92 medical device manufacturers.
The State Food and Drug Administration has requested the food and drug supervision and administration department of the locality to follow the "Regulations on the Supervision and Administration of Medical Devices" and the General Office of the Food and Drug Administration on further strengthening the inspection of medical devices. Notice of the Food and Drug Administration (Farming Supervision [2016] No. 9), investigate and deal with relevant enterprises.
Relevant medical device manufacturers shall conduct risk assessments for products that do not meet the standards and products that do not meet the standards, determine the recall level according to the severity of the medical device defects, and actively recall and publicly recall the information. The provincial food and drug supervision and administration department of the enterprise shall supervise the recall of the enterprise, and shall not recall the recall of the organization; if it is found that the medical device product does not meet the standards and cause harm to the human body or there is evidence that it may endanger human health, it shall be taken. Suspension of emergency control measures for production, import, operation and use. Relevant provincial food and drug supervision and administration departments should urge enterprises to ascertain the reasons as soon as possible, formulate corrective measures and rectify them on schedule, and the relevant disposals will be announced to the public before July 21, 2017.
- ã€Collection of this page】 ã€Copy Link】 ã€Print】
Fresh White Garlic,Hardneck Garlic,White Garlic Plant,Fresh White Garlic In Bulk
shandong changrong international trade co.,ltd. , https://www.cragriculture.com