ObsEva's gynecological drug Linzagolix releases 2b positive data
ObsEva's gynecological drug Linzagolix releases 2b positive data
June 20, 2018 Source: Sina Pharmaceutical
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Founded in 2012 and headquartered in Geneva, Switzerland, ObsEva SA is a clinical stage biopharmaceutical company focused on the development and commercialization of new therapies for women aged 15 to 49 with reproductive health and pregnancy disorders.
Recently, the company announced the 12-week positive test results of the EDELWEISS phase 2b clinical trial of the drug linzagolix (OBE2109, oral GnRH receptor antagonist), which is mainly used to treat pain associated with endometriosis. Currently, ObsEva's candidate products include: OBE2109 is an oral gonadotropin-releasing hormone receptor antagonist for the treatment of pain associated with endometriosis and severe menstrual bleeding associated with uterine fibroids in premenopausal women; OBE001 is an oral oxytocin receptor antagonist to improve clinical pregnancy and live birth rate in women receiving IVF; OBE022 is an oral and selective prostaglandin F2a receptor antagonist once daily for pregnancy Prevention of preterm birth 24 to 34 weeks.

The EDELWEISS Phase 2b trial is a randomized, double-blind, placebo-controlled clinical trial designed to assess the safety and efficacy of multiple doses of linzagolix in 327 women from 64 gynaecological clinics in the US and Europe. Recruited in both, and all have moderate to severe endometriosis-related pain. In the trial, the subjects first determined the baseline pain level during the introduction phase of the two menstrual cycles, then randomized the patients, and received a daily linzagolix oral dose (50 mg, 75 mg, 100 mg or 200 mg) or placebo. And record 12 weeks of treatment results.
The primary end point of the EDELWEISS clinical trial was the treatment response analysis, which was defined as a reduction in menstrual and non-menstrual combined pelvic pain by at least 30%, and a daily oral rating scale (VRS) of 0 for the past 28 days recorded by electronic diary (0). No pain) to 3 (severe pain). The trials showed an average baseline overall pain VRS of 1.7, a menstrual pain VRS of 2.1, and a non-menstrual pain VRS of 1.6, with population and baseline characteristics comparable. In addition, through the endometriosis health file 30 score, the patient's overall impression change scale (PGIC), the patient's overall impression severity (PGIS), the patient's overall impression scale assessment results, 75mg to 200mg dose of linzagolix can be significant The patient's insomnia symptoms and activity disorder scores, Biberoglu and Behrman scores were consistently improved. All doses of linzagolix also improved the rate of misdiagnosis and reached statistical significance at the 200 mg therapeutic dose.
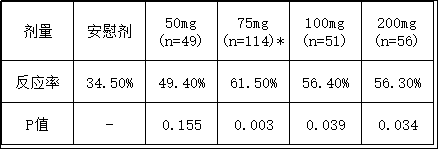
After 12 weeks of linzagolix treatment, the median level of serum estradiol was 12 pg/ml in the 200 mg dose and 48 pg/ml in the 75 mg dose, indicating that the estradiol was completely inhibited after receiving a higher dose of linzagolix at 75 mg. Partial inhibition was obtained at the time of the dose. The trial also showed that linzagolix has good safety and tolerability. Depending on the treatment classification and mechanism of action, a moderate proportion of patients reported at least one hot flash event (inhibition of side effects caused by E2 levels in serum). The incidence of hot flashes in the 75 mg dose cohort was 18.4%, and the incidence of hot flashes in the 200 mg dose cohort was 42.1%, compared with 10.9% in the placebo group.
Ernest Loumaye, CEO and co-founder of ObsEva, OB/GYN, said, "I believe these data strongly support linzagolix to improve the condition and health potential of patients with endometriosis. In addition, the data further confirms ObsEva The vision and product development strategy, that is, a significant proportion of patients do not require complete estradiol inhibition, which requires the use of supplemental hormone replacement therapy. Based on these data, we intend to test the two doses of linzagolix before the end of the year." Dr. Hugh Taylor, director of the Department of Obstetrics and Gynecology and Reproductive Sciences at Yale University School of Medicine and director of the Department of Obstetrics and Gynecology at Yale University's New Haven Hospital, commented: "There are many women with endometriosis who are diverse and diverse, and who have new treatments. The huge demand, it is encouraging to see that linzagolix may offer a range of effective medications to meet the individual needs of patients."
Currently, patients in the EDELWEISS trial will continue to receive additional linzagolix for 12 weeks, and other data including bone mineral density (BMD) assessment are expected to be available in the fourth quarter of 2018. ObsEva then intends to seek feedback from the regulatory body to obtain advice from the regulatory body on the design of Phase 3 clinical trials, which will be launched by the end of 2018. (Sina Pharmaceutical Compilation / Fan Dongdong)
Article Reference Source: ObsEva SA Achieves Primary and Secondary Endpoints for EDELWEISS Phase 2b Clinical Trial of Linzagolix (OBE2109) in Women with Endometriosis
Food additives are substances added to food. Some additives have been used for centuries. With the advent of processed foods in the second half of the twentieth century, many more additives have been introduced, of both natural and artificial origin.
The advantages of food additives are these followed:
1. Improve the appearance of food.
2. Keep and enhance the nutrition value of food.
3. Increase the variety and conveniences of food.
4. Miantain freshness and prolong shelf life.
5. Provide leavening or control acidity and alkalinity.
Food Additives,Natural Food Additives,Food Additives Preservatives
SINOCHEM PHARMACEUTICAL CO., LTD , https://www.sinochemnutrition.com