FAO pathway promotes distant metastasis of colorectal cancer cells by inhibiting anoikis
Professor Xu Ruihua from the Cancer Research Institute of the Affiliated Tumor Hospital of Sun Yat-sen University has been engaged in the research of individualized treatment of gastrointestinal cancer and anticancer drugs. The team used the NuRNATM Human Central Metabolism PCR Array study to find that fatty acid oxidation (FAO) pathways are activated in non-adherent colorectal cancer cells. In metastatic tumors, CPT1A expression is up-regulated, activated by CPT1A-mediated FAO pathway, inhibits anoikis by regulating redox homeostasis, and promotes proliferation of non-adherent CRC cells. The research was published in the academic journal Oncogene ( IF: 6.854) .
Research Background
Colorectal cancer (CRC) is the most common gastrointestinal tumor, which is the leading cause of tumor-related death due to its prone to distant metastasis. Distal metastasis is a complex process in which aggressive cancer cells detach from the primary tumor and enter the blood and lymphatic vessels. Only some of these cells can ooze out of the blood vessels and settle in new organs, forming a distant metastasis. Most of the cells in the metastasis process lose their adhesion to the extracellular matrix due to detachment from the primary tumor, resulting in anoikis.
FAO, also known as beta-oxidation, is the process by which fatty acids decompose to produce acetyl-CoA, while producing ATP, NADPH, MADH and FADH 2 . Carnitine palmitoyltransferase 1A (CPT1A) is localized on the mitochondrial membrane and is the most important rate-limiting enzyme in FAO. It has been reported that CPT1A is associated with a variety of tumor processes. Considering that FAO is involved in the metabolic process of non-adherent tumor cells, the authors speculate that CPT1A is a good target for tumor cell energy metabolism regulation and metastasis inhibition.
Research ideas:
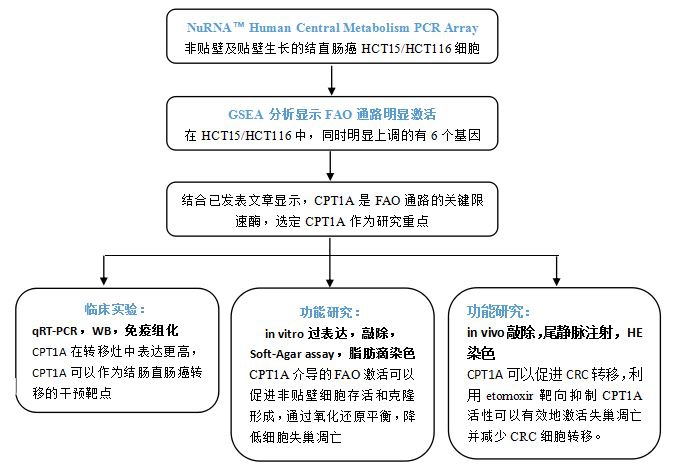
This study focuses on CPT1A-mediated activation of the FAO pathway and protects colorectal cancer cells from anoiki by maintaining redox homeostasis. The authors used NuRNATM Human Central Metabolism PCR Array to analyze the expression of metabolic related genes in ultra-low adhesion growth and normal adherent growth of HCT15 and HCT116 cells. Gene enrichment analysis showed that the FAO pathway was significantly activated in both cells. Since CPT1A is a key gene in the FAO pathway, the authors therefore focused on the role of CPT1A in CRC metastasis. qRT-PCR results showed that key genes in the FAO pathway were significantly up-regulated in suspended CRC cells. Later, the authors analyzed samples of orthotopic tumors and metastases in clinical patients and found that CPT1A was up-regulated in metastases.
To further investigate the function of CPT1A in non-adherent CRC cells, the authors established a stable strain of CPT1A knockdown, and found that knockdown of CPT1A significantly inhibited cell survival and colony formation when cells were non-adherently grown, and could also be detected. Cell anoikis are significantly elevated. The addition of oleate to the medium promotes the formation of CRC cell clones, which can be inhibited by etomoxir, suggesting that CPT1A-mediated FAO activation promotes proliferation of non-adherent CRC cells.
In CPT1A knockdown CRC cells, the balance of NADPH/NADP+ and GSH/GSSG could not be maintained, resulting in high levels of reactive oxygen species. Therefore, CTP1A-induced FA is the main factor inhibiting anoikis by promoting active oxygen scavenging. In vivo, knockdown of CPT1A can reduce liver metastasis and lung metastasis of CRC cells, and etomoxir can inhibit CRC liver metastasis and lung metastasis, indicating that targeting ettoxir targeting CPT1A can effectively activate anoikis and reduce CRC cells. Transfer.
Technical route :
The results show :
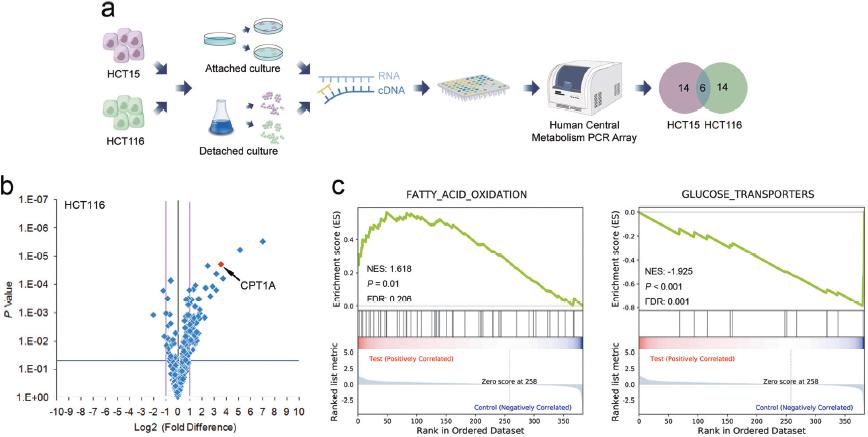
Figure 1: a experimental procedure; b\c NuRNATM Human Central Metabolism PCR Array analysis of volcano maps and gene enrichment analysis of metabolically related gene transcriptional changes;
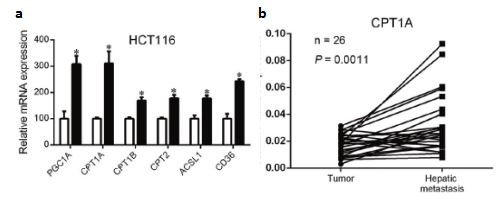
Figure 2: a qPCR to verify that the FAO pathway-related genes are highly expressed in non-adherent HCT116, the FAO pathway is activated; b CPT1A is up-regulated in metastases
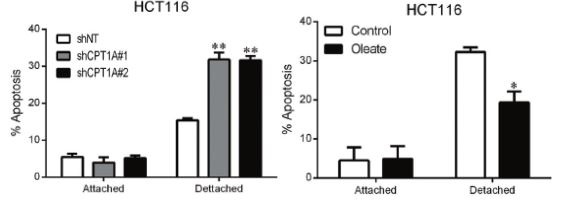
Figure 3: Knockout of CPT1A in non-adherent HCT116 cells or the addition of oleate to the medium to promote anoikis
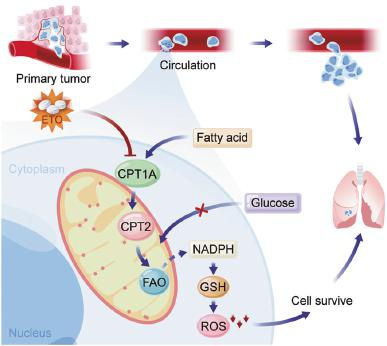
Figure 4: Summary: CRC cells entering the blood, due to the upregulation of CPT1A expression, activate the FAO signaling pathway, providing sufficient reducing power for the transferred cells, allowing the cells to overcome reactive oxygen stress and ultimately survive at the metastatic site.
Research significance :
This study screened CPT1A, which is highly expressed in non-adherent colorectal cancer cells, by NuRNATM Human Central Metabolism PCR Array, and found that CPT1A-mediated activation of fatty acid oxidation pathway protects CRC cells from phagocytosis by maintaining redox homeostasis. Death, promote survival. It is predicted that targeting CPT1A will be a promising clinical application in patients with metastatic CRC.
 Â
Original source :
CPT1A-mediated fatty acid oxidation promotes colorectal cancer cell metastasis by inhibiting anoikis. Oncogene. 2018
Lpcb Certified,Fire Alarm Components And Linkage Components,Fire Smoke Alarm System,Intelligent Smoke Alarm System,Fire Alarm Programmer
LIAONING YINGKOU TIANCHENG FIRE PROTECTION EQUIPMENT CO.,LTD , https://www.tcfiretech.com