Application overview: Measuring the particle size of a small amount of dry powder using Aero S and Mastersizer 3000
2023-06-05 07:15:11
Conventional particle size measurement plays an important role in the pharmaceutical industry and other industries and is key to ensuring product quality. Dispersion is critical for any particle size measurement process, and there are two main approaches in laser diffraction systems: wet and dry. If the powder in the dry state is treated and the final product is a dry powder, the dry dispersion method is generally preferred. In the pharmaceutical industry, the active ingredients and excipients are usually treated in a dry form and the final state may be a dry powder, such as a dry powder inhaler. In the early stages of product development, pharmaceutical manufacturers may wish to measure the particle size of dry powder samples using less than 100 mg of existing materials.
In this application note, we used only 5 mg of fine medicinal lactose aliquots for measurement and achieved good measurement reproducibility – in accordance with the requirements of the ISO Laser Diffraction Standard [1] guidelines for reproducibility. We chose fine lactose as the active ingredient of the drug and a high-adhesive powder modeling material that is difficult to disperse.
Measuring fine lactose
Aliquots of aliquots of lactose are weighed out and carefully treated to prevent the powder from building up or adhering to the weighing pan. Choose 5mg aliquots so that you can take 20 measurements using the existing 100mg to provide enough aliquots for both method development and reproducibility testing.
Reproducibility of 5mg aliquots
Six aliquots of fine lactose were prepared and dispersed using Aero S. A typical particle size distribution is shown in Figure 1. The resulting variability (or reproducibility) of Dv (50) was less than 0.8% (Table 1A). The ISO standard [1] requires that the variability (RSD%) between Dv (50) repeated measurements should be less than 3%. Since fine lactose is a highly adherent material, such a low degree of variability can be achieved between repeated measurements, demonstrating the excellent dispersion properties of Aero S.
Mass titration
Mass titration was performed to correlate 5 mg aliquots with most of the materials and confirm that the 5 mg results are typical. Three equal parts were measured for 5 mg, 10 mg, 20 mg, 50 mg and a large portion (about one teaspoon). See Figure 2 for a comparison of particle size distribution. The graph must be significantly enlarged to distinguish between measurements. This shows a very high agreement between the measurements. For all three percentage values, the variability (quantified by RSD) between aliquot measurements of different masses was less than 1.5% (Table 1B). This is in full compliance with the requirements of the ISO standard [1] guidelines for repeated measurements of the same sample. If the reproducibility data allows the RSD to fully comply with the reproducibility requirements of the ISO guidelines, it is clear that Aero S is able to consistently disperse samples of different quality.
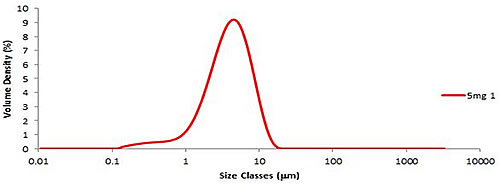
Figure 1: Size distribution of the first 5 mg aliquot of fine lactose.
Table 1A: Variability of six 5 mg aliquots of fine lactose (determined by RSD);
A | DV (10) (μm) ISO 5% | DV (50) (μm) ISO 3% | DV (90) (μm) ISO 5% |
5mg 1 | 1.38 | 3.93 | 8.40 |
5mg 2 | 1.36 | 3.89 | 8.25 |
5mg 3 | 1.36 | 3.91 | 8.19 |
5mg 4 | 1.36 | 3.88 | 8.26 |
5mg 5 | 1.39 | 3.95 | 8.33 |
5mg 6 | 1.36 | 3.96 | 8.43 |
1RSD (%) | 0.949 | 0.795 | 1.11 |
Table 1B: Variability of percentage values ​​between aliquots of different masses.
B | DV (10) (μm) ISO 5% | DV (50) (μm) ISO 3% | DV (90) (μm) ISO 5% |
5mg | 1.38 | 3.93 | 8.40 |
10mg | 1.37 | 3.92 | 8.38 |
20mg | 1.38 | 3.99 | 8.54 |
50mg | 1.39 | 3.99 | 8.52 |
Large part | 1.41 | 4.06 | 8.61 |
1RSD (%) | 1.14 | 1.37 | 1.15 |
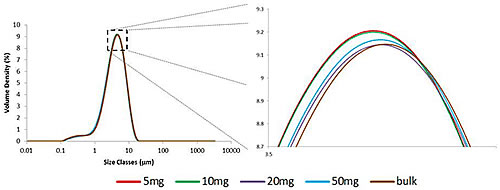
Figure 2: Overlapping particle size distribution obtained from samples of different masses. The distribution profiles are very close and must be magnified significantly to distinguish them.
The importance of sampling and the impact of granularity
In addition to achieving good dispersion, the reproducibility of small aliquot results will also depend on the median size and distribution range. If the median particle size is small, as in this case, even 5mg also contains hundreds of particles. Assuming the sample has been mixed, we can be confident that the 5 mg sample here will represent the entire sample. As the particle size increases, the number of particles in the sample will decrease to a few hundred, and a 5 mg sample may not be sufficient to represent most of the sample. For example, if a 5 mg aliquot is used to measure a crude lactose with a Dv (50) of 100 μm, the resulting percentage value will have a higher variability (indicating a more difficult sampling). Therefore, it may not be appropriate to use such a small aliquot to measure the crude material. Similarly, if the distribution range is large (indicating that the sample is a polydisperse sample), the mass of the aliquot is limited by the sampling capacity of the more distributed end.
To determine the quality required to implement a reproducible measurement, a mass titration similar to that described above should be performed. The minimum aliquot is the minimum quality when the RSD meets the requirements of the ISO Standard [1] guidelines.
to sum up
Certain industries, including the pharmaceutical industry, require measurements of very small amounts of dry powder. We measured the 5 mg drug material model per aliquot using the Aero S dry powder dispersion unit and the Mastersizer 3000. We found that the variability of the results obtained with 5 mg was less than 1% RSD, which indicates that Aero S has excellent fine powder dispersion properties. The result of 5 mg per aliquot is very consistent with the results obtained from the large measurement.
[1] ISO 13320:2009 "Particle size analysis - laser diffraction method".
Innovative jet embedded nylon technology maximizes the efficiency of sample collection from patients. Nylon adheres vertically and evenly to the surface of the swab tip, improving the efficiency of cell and liquid sample collection and release. Increased analytical sensitivity, no specimen residue, and accelerated specimen processing.
Flocked Swab,Collect swabs from specimens with flocked tips,Medical sampling swab oral nylon swab flocking,Acts in the clinical diagnosis
Jiangsu iiLO Biotechnology Co., Ltd. , https://www.sjiilobiotech.com