A new mechanism by which mitochondria regulate protein homeostasis in cells
A new mechanism by which mitochondria regulate protein homeostasis in cells
January 04, 2019 Source: Animal Research Institute
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Quality control of proteins and mitochondria in organisms is critical to the maintenance of cellular vitality. Protein homeostasis in cells is primarily maintained by the coordinated operation of the chaperone protein system with two proteolytic systems, the ubiquitin-proteasome system and the autophagy-lysosomal system. As a center of energy and metabolism of cells, mitochondria have relatively independent quality control systems, including molecular level oxygen free radical scavenging systems, molecular chaperone protein systems and protease systems, as well as organelle-level fusion/splitting mechanisms and mitochondrial autophagy mechanisms. The imbalance of protein homeostasis and mitochondrial dysfunction are important factors in the development of aging and aging-related diseases. The occurrence of the two may be mutually causal, but the specific mechanisms of their interconnection are still unclear.
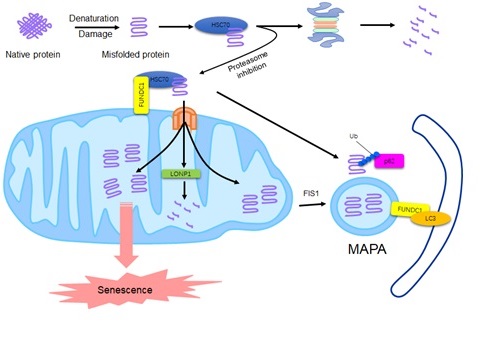
Researchers at the Institute of Zoology of the Chinese Academy of Sciences have found that the mitochondrial autophagy receptor protein FUNDC1, which localizes to the mitochondrial outer membrane, interacts with the molecular chaperone protein HSC70 localized to the cytosol. Damaged or misfolded proteins in the cytosol can be recruited to the mitochondria by this interaction, followed by TOM-TIM complexes entering the mitochondrial matrix and being degraded by the mitochondrial protease LONP1 localized to the matrix. When the activity of the proteasome in the cell is inhibited, the interaction between FUNDC1 and HSC70 is enhanced, and the unfolded protein entering the mitochondria is correspondingly increased. If these proteins are not removed in time in the mitochondrial matrix, they will participate in the formation of a specific multilayer membrane structure. With the participation of the mitochondrial division-associated protein FIS1, these multilayer membrane structures are separated from the mitochondrial network and form Mitochondrion Associated Protein Aggregates (MAPAs). MAPA differs from previously reported Aggresome, which contains mitochondria-derived proteins, including the mitochondrial membrane protein FUNDC1. FUNDC1 mediates autophagic degradation of MAPA through interaction with LC3. This new pathway, mediated by FUNDC1 and HSC70, can help cells maintain protein homeostasis by enabling the mitochondrial self-protease system or mitochondrial autophagy, but excessive accumulation of unfolded proteins on mitochondria can impair mitochondrial integrity and activation. AMPK also causes cell aging to occur. Therefore, this compensatory degradation of mitochondria-mediated proteins is at the expense of the normal function of the mitochondria itself and the health and vitality of the cells. These findings establish a new link between protein stability imbalance, mitochondrial dysfunction, and cellular senescence, providing new explanations for the causes of aging and aging-related diseases, and providing new theoretical guidance for the treatment and prevention of these diseases. .
The research results were published in the EMBO Journal. Postdoctoral Li Yanjun is the first author of the thesis. Researcher Chen Wei and associate researcher Liu Lei are the authors of the paper. The research was funded by the National Natural Science Foundation of China, the Special Fund for Strategic Pilot Science and Technology of the Chinese Academy of Sciences, the National Key Research and Development Project of the Ministry of Science and Technology, the Key Science Program of the Chinese Academy of Sciences, and the China Postdoctoral Science Foundation.
A new mechanism by which mitochondria regulate protein homeostasis in cells
We, Jiangsu YanFang Medical Technology Co., Ltd, commenced our medical gloves manufacturing in 2020. Currently, we possess a total of 12 high-capacity NBR Glove Dipping Production Lines.
Likewise, we are not only certified with ISO9001, ISO13485 but also fully complied with the essential USFDA, CE Compliances, as well as obtaining relevant accreditation of FDA510K, EN455, and EN374.
Nonetheless, our NBR Examination, Chemotherapy, and Food Grade products are being well established in both US and Europe markets.
We look forward to cooperating and working closely with our valuable customers and stakeholders, who are seeking long-term business relationships in high-quality NBR glove supplies.
Medical Examination Nitrile Gloves,Disposable Nitrile Gloves,Powder Free Nitrile Gloves,Non-sterile Nitrile Gloves
Jiangsu Yanfang Medical Technology Co.,Ltd. , https://www.yanfangchina.com